By all measures, last year’s flu season was a mild one that peaked in early March. The number of cases reported, the total number of pneumonia/influenza deaths, and the number of pediatric deaths were all comparatively low.1 The main circulating strains were the same as the previous 2 years, leaving much of the population protected by immunity gained from natural infections. And there was a good match between the vaccine and the circulating strains, leading to higher vaccine effectiveness.
So what’s new—and what remains the same—for the 2012-2013 season? Annual flu vaccination continues to be recommended for everyone ≥6 months of age, starting as soon as the vaccine is available and continuing through the influenza season. This season’s vaccine contains 2 new strains in addition to the A/California/7/2009 (H1N1) strain that has been in the vaccine the past 2 seasons and was used for a monovalent vaccine in 2009. The new strains are A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010.2
Each year, influenza experts decide which strains to include in the vaccine based on the ones currently circulating and the ones expected to circulate into the next season. The degree to which vaccine strains match circulating strains will determine the effectiveness of the vaccine.
A variety of products
The number of influenza vaccine products has increased, and they differ in the ages for which they are approved.2 All trivalent inactivated vaccine (TIV) products are given intramuscularly, with the exception of a relatively new product, Fluzone Intradermal (Sanofi Pasteur). There is also FluMist (MedImmune), a live-attenuated influenza vaccine (LAIV), administered as a nasal spray. And, for adults ≥65 years, the product Fluzone High-Dose contains 4 times the antigen content of other vaccines for this age group. The Centers for Disease Control and Prevention (CDC) does not express a preference for any one of the TIV products over the others.
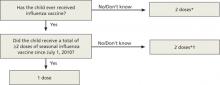
A new algorithm for children <9 years
To mount an adequate immune response in children <9 years, 2 doses of vaccine are needed in the first year of receiving vaccine. The time between doses should be at least 4 weeks; longer intervals do not appear to matter. This leads to a complex algorithm when taking into consideration vaccines given in past years. To simplify the issue, the CDC has approved an algorithm for this season that involves only 2 questions (FIGURE 1).
However, strict adherence to this algorithm will result in some children receiving 2 doses when one would suffice. If a child has received at least 2 doses of seasonal influenza vaccine in any prior season, and at least one dose of A/California/7/2009 (H1N1), then just a single dose of vaccine is needed this year. The A/California antigen could have been in the monovalent product in 2009 or the regular trivalent products in the past 2 seasons. If any doubt or confusion exists, administer 2 doses, 4 weeks apart. There is no harm in receiving 2 doses if only one is needed.
FIGURE 1
The CDC’s new dosing algorithm for children 6 months–8 years receiving influenza vaccine in the 2012-2013 flu season2
*Doses should be administered at least 4 weeks apart.
†For simplicity, this algorithm takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. However, if a child 6 months through 8 years of age is known to have received at least 2 seasonal influenza vaccines during any prior season, and at least one dose of a 2009 (H1N1)-containing vaccine-—ie, either 2010-2011 or 2011-2012 seasonal vaccine or the monovalent 2009 (H1N1) vaccine—then the child needs only one dose for 2012-2013.
Egg allergy precautions
While all influenza vaccines are manufactured by injecting virus into chicken eggs, the amount of egg protein in the vaccine is extremely low. Those who react to egg products only with hives can receive TIV (not LAIV) under the care of a clinician who is familiar with egg allergy manifestations. (Patients should be observed for 30 minutes after vaccine administration.) Those who have more severe reactions to eggs (FIGURE 2) require assessment by a physician with allergy expertise before receiving an influenza vaccine. All facilities that administer vaccines should be equipped to respond to anaphylaxis, and all providers who administer vaccines should be adequately trained in anaphylaxis management. The CDC made these recommendations last year, and there was no increase in egg allergy adverse events following vaccination reported during the 2011-2012 flu season.3