For Medicare beneficiaries with rheumatoid arthritis, out-of-pocket costs are closely linked to whether a patient is more likely to receive facility- or home-administered biologic treatment, according to a retrospective analysis of how patient cost sharing affects utilization and spending patterns.
Medicare Part D beneficiaries with rheumatoid arthritis (RA) who receive a low-income subsidy (LIS) are more likely to receive biologic medication that can be self-injected at home, the study found, while those without subsidies face greater out-of-pocket costs for outpatient medications and are more likely to receive facility-administered drugs. Lead study author Dr. Jinoos Yazdany noted, “Our findings shed light on the complexity of current Medicare drug coverage policies for biologic DMARDs.”
Dr. Yazdany and her associates at the University of California, San Francisco, used 2009 Medicare claims to obtain a random nationwide sample of 5% of all Medicare beneficiaries with RA who were dispensed at least one RA medication. Of the 6,932 beneficiaries identified, 1,812 (26.1%) were dispensed a biologic disease-modifying antirheumatic drug (DMARD). Utilization patterns of the remainder, who received a nonbiologic DMARD, were used for comparison. In 2009, the biologic DMARDs infliximab, abatacept, and rituximab were all facility-administered medications reimbursable under Medicare Part B, while etanercept, adalimumab, and anakinra were available for home self-injection and were therefore Part D drugs (Arthritis Care Res. 2015 March 16 [doi:10.1002/acr.22580]).
Examining biologic DMARD use and costs, the study calculated costs to Medicare for both home- and facility-administered medications. Part D dispensing data were used to calculate costs to patients by determining the actual out-of-pocket dollar costs for beneficiaries. This information was not available for Part B events, so investigators used a range from 0%-20% cost-sharing to estimate patient costs.
Patient costs for Medicare Part D were further divided by LIS status, since LIS confers minimal cost sharing for outpatient drugs. By contrast, nonsubsidized Part D recipients incur variable costs; the current Medicare Part D coverage gap – also known as the doughnut hole – means that up to 100% of the cost of medications may be borne by the patient at certain points in the coverage cycle.
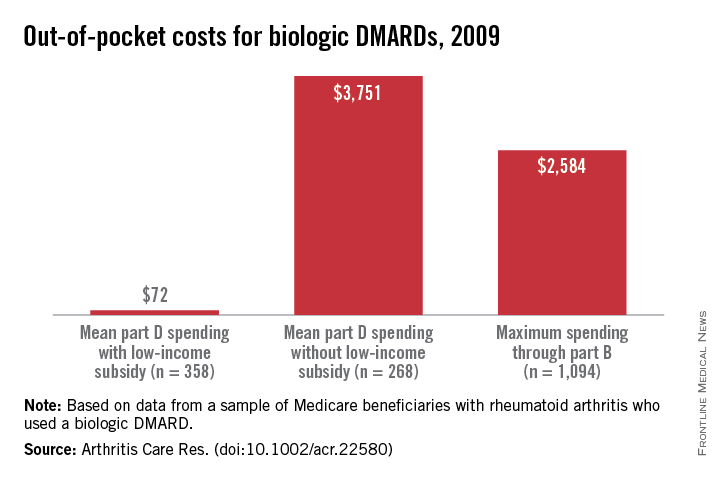
Multinomial regression analysis showed that those receiving a facility-administered biologic DMARD under Part B were significantly less likely than those receiving nonbiologic DMARDs to have a LIS (relative risk, 0.58; 95% confidence interval, 0.48-0.69). Recipients of self-injected DMARDs under Part D, in contrast, were much more likely to have a LIS than were those in the nonbiologic DMARD group (RR, 2.98; 95% CI, 2.50-3.56). Annual Part D out-of-pocket costs for those receiving biologics with no LIS were a mean $3,751, compared with a mean $72 for the LIS Part D group. However, beneficiaries with no LIS paid a maximum of $2,584 out of pocket for biologic DMARDs through Part B (assuming 20% supplemental insurance coverage).
Since the Affordable Care Act will reform Part D to help close the coverage gap, the study also projected how ACA reform, once adopted, would affect cost sharing and use patterns. Dr. Yazdany and her colleagues projected out-of-pocket biologic DMARD costs into full reform implementation in the year 2020, calculating that nonsubsidized out-of-pocket expenses would still be $2,588. The authors noted that “patient cost sharing is an important determinant of drug choice in Medicare’s part B versus part D programs ... the current system risks significant financial burden to many patients.”
When asked to comment on the study, Kaleb Michaud, Ph.D., assistant professor of rheumatology at the University of Nebraska Medical Center, Omaha, and codirector of the National Data Bank for Rheumatic Diseases, noted that this well-constructed study made very good use of Medicare data to point to a fundamental problem with reimbursement for rheumatic disease medications. “Rather than asking, ‘What’s the best drug for this patient?’ we find ourselves asking, ‘What drug can this patient afford?’ ” he said.
The study was funded by the National Institutes of Health, a Research Allocation and Evaluation Award from the University of California, San Francisco, and the Rosaline Russell Medical Research Center for Arthritis. The authors reported that they had no conflicts of interest.