› Individualize treatment regimens based on severity of symptoms and risk for exacerbation, prescribing short-acting beta2-agonists, as needed, for all patients with chronic obstructive pulmonary disease (COPD). A
› Limit use of inhaled long-acting beta2-agonists to the recommended dosage; higher doses do not lead to better outcomes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
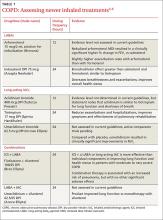
Chronic obstructive pulmonary disease (COPD) carries a high disease burden. In 2012, it was the 4th leading cause of death worldwide.1,2 In 2015, the World Health Organization updated its Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, classifying patients with COPD based on disease burden as determined by symptoms, airflow obstruction, and exacerbation history.3 These revisions, coupled with expanded therapeutic options within established classes of medications and new combination drugs to treat COPD (TABLE 1),3-6 have led to questions about interclass differences and the best treatment regimen for particular patients.
Comparisons of various agents within a therapeutic class and their impact on lung function and rate of exacerbations address many of these concerns. In the text and tables that follow, we present the latest evidence highlighting differences in dosing, safety, and efficacy. We also include the updated GOLD classifications, evidence of efficacy for pulmonary rehabilitation, and practical implications of these findings for the optimal management of patients with COPD.
But first, a word about terminology.
Understanding COPD
COPD is a chronic lung disease characterized by progressive airflow limitation, usually measured by spirometry (TABLE 2),3 and chronic airway inflammation. Emphysema and chronic bronchitis are often used synonymously with COPD. In fact, there are important differences.
Individuals with chronic bronchitis do not necessarily have the airflow limitations found in those with COPD. And patients with COPD develop pathologic lung changes beyond the alveolar damage characteristic of emphysema, including airway fibrosis and inflammation, luminal plugging, and loss of elastic recoil.3
The medications included in this review aim to reduce both the morbidity and mortality associated with COPD. These drugs can also help relieve the symptoms of patients with chronic bronchitis and emphysema, but have limited effect on patient mortality.
Short- and long-acting beta2-agonists
Bronchodilator therapy with beta2-agonists improves forced expiratory volume in one second (FEV1) through relaxation of airway smooth muscle. Beta2-agonists have proven to be safe and effective when used as needed or scheduled for patients with COPD.7
Inhaled short-acting beta2-agonists (SABAs) improve FEV1 and symptoms within 10 minutes, with effects lasting up to 4 to 6 hours; long-acting beta2-agonists (LABAs) have a variable onset, with effects lasting 12 to 24 hours.8 Inhaled levalbuterol, the last SABA to receive US Food and Drug Administration approval, has not proven to be superior to conventional bronchodilators in ambulatory patients with stable COPD.3 In clinical trials, however, the slightly longer half-life of the nebulized formulation of levalbuterol was found to reduce both the frequency of administration and the overall cost of therapy in patients hospitalized with acute exacerbations of COPD.9,10
Recently approved LABAs
Clinical trials have studied the safety and efficacy of newer agents vs older LABAs in patients with moderate to severe COPD. Compared with theophylline, for example, formoterol 12 mcg inhaled every 12 hours for a 12-month period provided a clinically significant increase of >120 ml in FEV1 (P=.026).11 Higher doses of formoterol did not provide any additional improvement.
In a trial comparing indacaterol and tiotropium, an inhaled anticholinergic, both treatment groups had a clinically significant increase in FEV1, but patients receiving indacaterol achieved an additional increase of 40 to 50 mL at 12 weeks.12
Exacerbation rates for all LABAs range from 22% to 44%.5,12,13 In a study of patients receiving formoterol 12 mcg compared with 15-mcg and 25-mcg doses of arformoterol, those taking formoterol had a lower exacerbation rate than those on either strength of arformoterol (22% vs 32% and 31%, respectively).10 In various studies, doses greater than the FDA-approved regimens for indacaterol, arformoterol, and olodaterol did not result in a significant improvement in either FEV1 or exacerbation rates compared with placebo.5,12,14
Studies that assessed the use of rescue medication as well as exacerbation rates in patients taking LABAs reported reductions in the use of the rescue drugs ranging from 0.46 to 1.32 actuations per day, but the findings had limited clinical relevance.5,13 With the exception of indacaterol and olodaterol—both of which may be preferable because of their once-daily dosing regimen—no significant differences in safety and efficacy among LABAs have been found.5,12,13